Novel Anticancer Agents – from Design to Clinical Translation
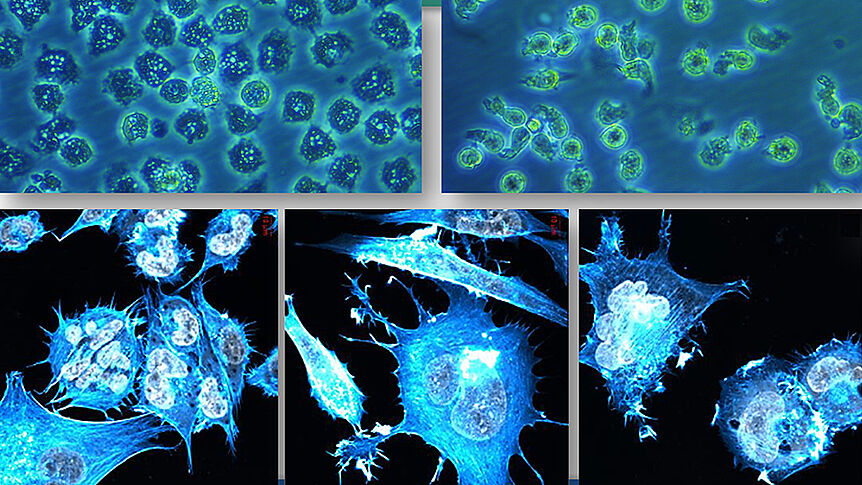
The fight against cancer is one of today's most urgent scientific tasks and of highest importance to public health. Despite a continuous improvement of the biomedical understanding of the genesis of cancer and the development of new therapeutic methods, every year 20.000 pepole are dying of cancer in Austria. Chemotherapy in all its forms is still the main therapy option for patients with metastasizing cancer, and metal-based drugs are an important part of the treatment of many types of cancer.
The Research-Platform Translational Cancer Therapy Research was founded in 2008 as a joint project of Bernhard Keppler from the Institute of Inorganic Chemistry, University of Vienna, and Walter Berger and Michael Micksche from the Center for Cancer Research, Medical University of Vienna, and was renewed as an Inter-University Clusterproject in 2016. The aim of the project is the development of new metal-based tumourtherapeutics as well as a better understanding of their modes of action. This information in turn allows for a systematic improvement of anti-cancer drugs and their optimisation for future clinical trials.
The Research Cluster promotes the intensive multidiciplinary collaboration between chemists, cell biologists and molecular biologists necessary for major advances in the fight against cancer. So far, three first-in-class compounds based on titanium, gallium and ruthenium have reached clinical studies; the ruthenium compounds IT-139 (KP1339) and LX-001 (KP46) are currently under development in USA and Canada.