The interdisciplinary Translational Cancer Therapy Research Cluster team headed by Prof. Bernhard Keppler (Institute of Inorganic Chemistry, University of Vienna) and Prof. Walter Berger (Center for Cancer Research and Comprehensive Cancer Center, Medical University of Vienna) has published a new study in the special issue “Metallodrugs for Targeted Cancer Therapy” of the scientific journal Pharmaceutics.
The presented study aimed at the dissection of mechanisms underlying the activity of the anticancer ruthenium complex BOLD-100 in colorectal and pancreatic cancer. Recently, BOLD-100 successfully completed a phase Ib clinical trial (NCT04421820) and is currently on the edge of global clinical phase II evaluation for the treatment of advanced gastrointestinal cancers in combination with the FOLFOX regimen. Since (chemo)therapy resistance remains a major hurdle in cancer treatment, another scope of the in January published research study was the investigation of mechanisms underlying acquired resistance against the ruthenium compound.
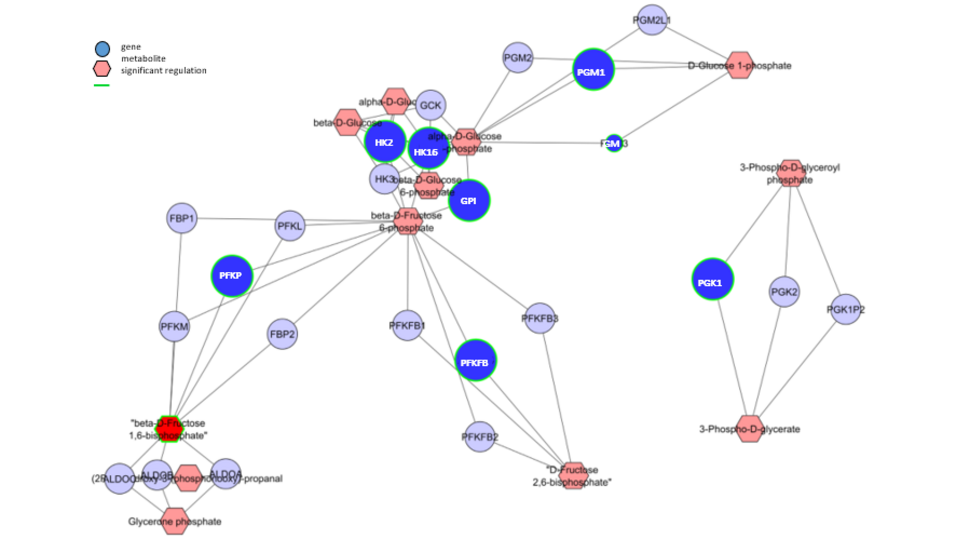
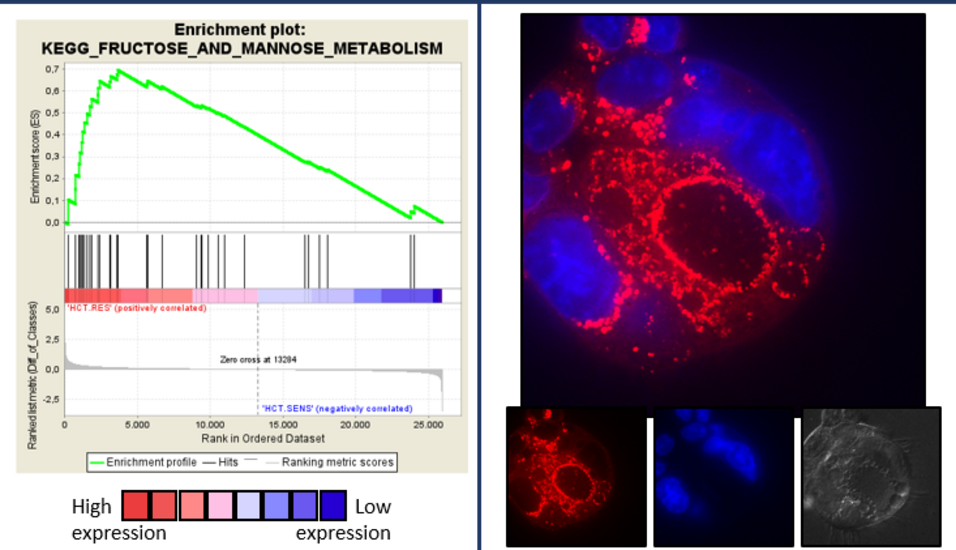
One highlight of the publication by Baier et al. is the identification of BOLD-100 as anti-Warburg drug, signifying glycolysis as novel target of the anticancer compound. Furthermore, the authors showed that development of resistance against the ruthenium complex was associated with enhanced glycolytic activity. This generated a metabolic vulnerability against glucose deprivation, which could be exploited therapeutically by using the glucose mimic 2-deoxyglucose. Interference with cellular glucose utilization efficiently caused death of acquired resistant cancer cells. Additionally worth to mention, inhibition of the cellular glucose metabolism further enhanced the anticancer activity of BOLD-100.
These findings open up a new perspective on the complex mode of action of BOLD-100 and shed light on potential new clinical considerations in the treatment of cancer using this unique and multifaceted metal compound.
- D. Baier, B. Schoenhacker-Alte, M. Rusz, C. Pirker, T. Mohr, T. Mendrina, D. Kirchhofer, S.M. Meier-Menches, K. Hohenwallner, M. Schaier, E. Rampler, G. Koellensperger, P. Heffeter, B. Keppler, W. Berger, The Anticancer Ruthenium Compound BOLD-100 Targets Glycolysis and Generates a Metabolic Vulnerability towards Glucose Deprivation, Pharmaceutics, 14 (2022) 238
- BOLD-100 (Website Bold Therapeutics)